

Frontline Learning Research Vol. 5 No. 3 Special
Issue (2017) 14 - 30
ISSN 2295-3159
aInstitut für Qualität und
Weiterbildung, Technische Hochschule Deggendorf, Germany
bSchool of Health Professions Education, Maastricht
University, the Netherlands
cDepartment of Cognitive Neuroscience Maastricht
University, the Netherlands
Article received 7 May / revised 23 March / accepted 23 March / available online 14 July
Functional neuroimaging is a useful approach to study the neural correlates of visual perceptual expertise. The purpose of this paper is to review the functional-neuroimaging methods that have been implemented in previous research in this context. First, we will discuss research questions typically addressed in visual expertise research. Second, we will describe which kinds of stimuli are employed and which functional-neuroimaging techniques are implemented in this kind of research, with a special focus on electroencephalography (EEG) and functional magnetic resonance imaging (fMRI). Third, we will summarize the outcomes of recent studies that addressed the neural correlates of visual expertise and will particularly focus on studies that examined the neural correlates of visual expertise in medical image diagnosis. Finally, the review closes with a discussion of the benefits, caveats, and future directions of cognitive-neuroscience research for studying visual expertise.
Keywords: Perceptual Expertise; EEG; fMRI; N170; FFA.
Expertise can be defined as maximal adaptations to task constraints (Ericsson & Lehmann, 1996; Gruber, Jansen, Marienhagen, & Altenmueller, 2010) which can take many forms, including, among others, motor expertise, memory expertise, or perceptual expertise (Ericsson & Lehmann, 1996). Perceptual expertise can be further categorized as visual, auditory, tactile, olfactory, vestibular, or gustatory expertise. Visual expertise is evident, for example, when bird experts classify a passing little bird as an oriole or a cardinal (Tanaka & Curran, 2001) or when clinicians diagnose digitized slides of human tissue as pathologically normal or abnormal (Helle et al., 2011). Assuming that individual differences in visual perceptual expertise should be reflected in differences in the brain, the following question arises: Can we reliably measure/objectify neural correlates of visual expertise with currently available functional-neuroimaging methods and therewith explain inter-individual behavioral differences with respect to visual perceptual expertise?
In line with the overall goal of this special issue to introduce and discuss methodological approaches in visual expertise research (Gegenfurtner & Van Merriënboer, 2017), the purpose of the present methodological review is to reflect on the promises and pitfalls of cognitive-neuroscience methods in the study of visual perceptual expertise. While the review can offer input for discussions among scholars experienced in conducting neuroscientific studies, the manuscript is mainly written to inform scholars who are unfamiliar with the methodological repertoire of functional neuroimaging and its use in expertise research. In this review, we will particularly address expertise in medical image diagnosis, which can be defined as the inspection and interpretation of a visual representation of the human anatomy or its functions (Gegenfurtner, Kok, Van Geel, De Bruin, Jarodzka, Szulewski, & Van Merriënboer, 2017); but because this body of research is still limited and in its infancy, we will extend our review to other content domains with the aim of offering a more useful overview of current methodological decisions in the visual perceptual expertise literature. There are already several systematic reviews available on the neural aspects of visual perceptual expertise (for example, Richler & Gauthier, 2014, for face perception or Gegenfurtner, Siewiorek, Lehtinen, & Säljö, 2013, for medical image diagnosis). The present review has a particular emphasis on implementing cognitive-neuroscience (especially functional-neuroimaging) methods on visual perceptual expertise, and will follow four steps. First, we will start with a short discussion of typically addressed research questions. Second, we will describe which kinds of stimuli are employed and which functional-neuroimaging methods are used, with a special focus on the frequently implemented methods EEG and fMRI. Third, we will summarize the outcomes of studies that addressed the neural correlates of visual perceptual expertise. And finally, we close this review with a discussion of the benefits, caveats, and future directions of cognitive-neuroscience research for studying visual expertise.
Research on visual perceptual expertise has focused on a wide range of different research questions. These research questions can be clustered in three distinct types: contrastive, developmental, and conditional. Naturally, research questions strongly correspond with the research design. For example, contrastive research questions ask how participants of different levels of expertise vary in different neural measures. In a classic study, Haller and Radue (2005) were interested in examining “neuronal activations during processing of radiologic and non-radiologic images by experienced radiologists and non-radiologist subjects by using event-related functional magnetic resonance (MR) imaging” (p. 983). This is a representative example for the first type of research questions (contrastive research questions). A second type of research questions, developmental research questions, asks how participants neurally adapt to visual perceptual training. These studies typically employ a paradigm implementing a training of inexperienced participants over the course of several weeks. For example, Gauthier and colleagues (1998) were interested in examining if increased experience with so-called ‘Greebles’, artificially created stimuli (see Figure 1), would yield to an increase of fMRI activation in a particular brain region, the so-called ‘fusiform face area’ (outcomes of this study presented and discussed below). Finally, the third type of research questions, conditional research questions, addresses the extent to which expertise effects – be they contrastive or developmental – are contingent on task conditions such as the duration of stimulus presentation or different manipulations of the presented stimuli. For example, Bilalić and colleagues (2016) were interested in unravelling if expertise effects are moderated by the orientation of the presented stimulus, in their case, X-ray films showed either in a normal, upright position or in an inverted position (rotated by 180°). Comparability across studies depends on the used research question and design. When designing a cognitive-neuroscience study, one can follow a single research question or several research questions even from different research-question types (see above). Typically, conditional research questions that address the moderating effect of stimulus or task conditions are often combined with contrastive or developmental research designs.
In this section, we review established methods implemented in cognitive-neuroscience studies in the field of visual perceptual expertise. We first describe frequently used artificial and naturalistic stimuli. We then look at the methodology of fMRI and EEG, in particular, on what kind of information can be derived from fMRI and EEG signals, and we also outline other, less frequently used techniques in cognitive neuroscience.
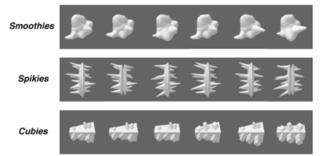
Figure 1. Examples of Smoothie, Spikie, and Cubie objects (open access from Op de Beeck et al., 2006).
3.1.1. Artificial stimuli
Artificially created stimuli are objects that have no common reference in the real world. This is a deliberate choice to avoid any confounding effects that may be induced from familiarity with the object. Several groups of artificial stimuli have been introduced. Some of those used more frequently are ‘Smoothies’, ‘Spikies’, and ‘Cubies’, and, perhaps most prominently, Greebles (mentioned above). Smoothies, Spikies, and Cubies are Matlab-generated classes of objects that “were designed to have different shape properties and to seem novel (i.e., they did not immediately suggest associations with everyday object categories” (Op de Beeck, Baker, DiCarlo, & Kanwisher, 2006, p. 13025). Figure 1 shows example Smoothies, Spikies, and Cubies. These artificial stimuli were created with variations of different dimensions, so that participants need to process more than one location of the object to attain high rates of discrimination.
Greebles are objects specifically constrained to be similar to faces along several dimensions. Figure 2 shows example Greebles. Greebles are photo-realistically rendered, three-dimensional, computer-generated objects that all share a common configuration. As Gauthier, Williams, Tarr, and Tanaka (1998) explain: “Each Greeble is made up of a vertically-oriented ‘body’ with four protruding ‘appendages’, from top to bottom, two ‘boges’ a ‘quiff’ and a ‘dunth’” (p. 2402). Greebles come in two different genders (called “glip” and “plok”) and five families (called “galli”, “osmit”, “radok”, “samar”, and “tasio”). Greebles have been used in a range of studies using both fMRI and EEG.
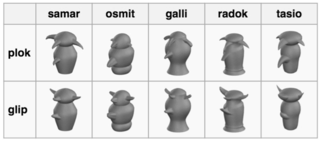
Figure 2. Examples of Greeble objects in their two genders and five families (open source from: https://commons.wikimedia.org/wiki/Category:Greeble).
3.1.2. Real-world stimuli
In opposition to these artificial stimuli that share little to no resemblance with naturally occurring objects, researchers also use real-world stimuli. These stimuli are called “real-world” to indicate that these objects are not researcher-generated. Associated with real-world stimuli is the assumption that there are real-world experts that have developed visual skills related to these objects (Shen, Mack, & Palmeri, 2014), so these material are used in an attempt to create ecologically valid domain-specific tasks. Real-world stimuli can be classified as faces and non-face objects. First, photographs of faces (or of parts of faces) are extensively used as stimuli in visual perceptual expertise research because we have so much exposure to faces that this makes us all experts in face recognition (Bentin, Allison, Puce, Perez, & McCarthy, 1996; Richler & Gauthier, 2014). Second, photographs of non-face objects includes cars (Gauthier, Skudlarski, Gore, & Anderson, 2000), different animal species such as birds (Tanaka, Curran, & Sheinberg, 2005) or dogs (Tanaka & Curran, 2005), and also letters such as Japanese (Maurer, Zevin, & McCandliss, 2008) and Chinese characters (Fan, Chen, Zhang, Qi, Jin, Wang, et al., 2015; Qi, Wang, Hao, Zhu, He, & Luo, 2016). Researchers also use representations of chess positions (Bilalić, Langner, Ulrich, & Grodd, 2011) and medical images (Haller & Radue, 2005). In many studies, these real-world stimuli are presented either in original form or in inverted, rotated, or otherwise artificially distorted. The rationale behind these artificial manipulations is to complicate and change pattern recognition for expert participants. Typically, real-world stimuli in these studies are static and two-dimensional. If we assume that the comprehension of visualizations is moderated by variations in dimensionality and dynamics (for a meta-analysis testing this assumption, see Gegenfurtner, Lehtinen, & Säljö, 2011), then it seems surprising that the literature on the neural correlates of real-world visual perceptual expertise has not yet systematically compared how the brain processes of experts and novices differ when they view static vs. dynamic stimuli or two-dimensional vs. three-dimensional visualizations.
While viewing different kinds of stimuli, participants’ neural correlates can be measured with cognitive-neuroscience techniques. Measuring these neural correlates is contingent on the study interests and research questions. Typically, if researchers are interested in the temporal aspects of image processing, they use electroencephalography (EEG). Conversely, if researchers are interested in the spatial aspects of image processing, they use functional magnetic resonance imaging (fMRI). In addition to EEG and fMRI, there are also several other measurement techniques, including magnetoencephalography (MEG), positron emission tomography (PET), and functional near-infrared spectroscopy (fNIRS). Offering detailed descriptions of each of these techniques is beyond the scope of this review. Ward (2006) and Squire and colleagues (2013) offer easy-to-understand introductions. But it is informative here to briefly describe the two most frequently used techniques to illustrate how they work and what they measure. These are EEG and fMRI.
3.2.1 EEG
Neurons communicate through electrical signals transmitted along axons and dendrites. When populations of neurons that are oriented in parallel are synchronously active, their electrical signals can be measured with electrodes placed on the scalp. Electroencephalography (EEG) records and amplifies these electrical signals over time. When we perceive a picture, particular populations of neurons in our brain respond to this picture. This response is measurable as a change in voltage at the scalp before, while and after seeing the picture. If we average the recorded EEG signal across many trials, random brain activity that is unrelated to the neural processing of the picture is cancelled out. The relevant (stimulus-related) signal is preserved and called the ‘event-related potential’ (ERP). When recording EEG from participants while they looked at pictures of faces, Bentin and colleagues (1996) found a negative event-related potential (N) that reached its maximum at approximately 172 ms (N170) after picture onset. Since this pioneering study, the N170 has become a widely studied EEG component in cognitive neuroscience. EEG measures have a high temporal resolution and are therefore time-sensitive. Thus, EEG can be especially used to investigate temporal patterns of brain activity. However, EEG has a relatively low spatial resolution meaning that the localization of the EEG signal source (i.e., the location of the specific neuronal populations evoking the electrical brain activity) cannot be ascertained with high precision.
3.2.2 fMRI
FMRI indirectly measures neural activity through its vascular response: Following (e.g., visual) stimulation, neuronal activity in particular (e.g., visual) brain regions increases which results in enhanced local oxygen consumption. Neuronal tissue gets new oxygen from the oxygenated hemoglobin in the blood. Within a few seconds, the blood flow and the concentration of oxygenated hemoglobin in the blood increases in the particular brain region. This increase is called the hemodynamic response. Since oxygenated and deoxygenated hemoglobin have different magnetic properties, the hemodynamic (or the blood oxygenation level-dependent, BOLD) response can be imaged using fMRI. Note that the hemodynamic response is considerably delayed and expanded which puts some constraints when designing fMRI experiments. Compared to EEG, the temporal resolution of fMRI is rather low (one data point is normally obtained within 1-2s). However, the spatial resolution is considerably higher (in the mm3 range) meaning that fMRI can provide specific information about the origin of the brain signal and therewith information about which part of the brain is involved in a particular activity (e.g., visual perception). Note that fMRI only measures relative (and not absolute) changes of the oxygenation level and that fMRI visualizations are actually representations of statistical differences of the fMRI signal across different experimental conditions.
In summary, EEG has a very high temporal resolution and is therefore a suited method to investigate timing of brain activity. FMRI has a much higher spatial resolution than EEG and is an appropriate method to indicate which brain regions are involved in a particular (e.g., perceptual) task.
This section presents the outcomes of studies addressing neural correlates of visual perceptual expertise. How does the development of expertise change temporal and spatial aspects of information processing? First, we summarize the findings of EEG research. Second, we review the fMRI findings. And finally, in a special section, we zoom in on the relatively new field of cognitive-neuroscience research applied to medical image diagnosis.
Using ERPs based on EEG measurements, cognitive-neuroscience research has provided strong support for the idea that a particular early ERP component, namely the N170 (introduced above), plays a significant role when participants process photographs and pictures of faces (Bentin et al., 1996; for a meta-analysis of this research, see Hinojosa, Mercado, & Carretié, 2015). Interestingly, it could be demonstrated that patients who suffer from face blindness, also called prosopagnosia (the inability to recognize faces), did not show this larger magnitude of the N170 component when processing faces (for reviews, see Richler & Gauthier, 2014; Towler, Fisher, & Eimer, 2017). This body of evidence on face processing has inspired research on perceptual expertise because of the assumption, in part, that all humans are ‘face experts’. If the N170 was such a stable neurophysiological marker in face perception, would the enhanced N170 also reflect expert processing of other familiar, domain-specific objects? A pioneering study by Tanaka and Curran (2001) confirmed this hypothesis. EEG was recorded while participants viewed photographs of cars or birds. Approximately 164 ms after stimulus onset, participants who were car experts showed a larger N170 component for cars compared to birds, and participants who were bird experts showed a larger N170 component for birds compared to cars. Tanaka and Curran (2001) carefully controlled for stimulus artefacts including image properties and task instruction, and also for group effects in that the same participants viewed photos of cars and birds and were thus expert and novice in different trials of the experiment. In summary, this study revealed that visual perceptual expertise is associated with an enhanced N170 component and therewith with very early stages of visual information processing. In recent years, this effect has been replicated with both car (Gauthier & Curby, 2005; Scott, Tanaka, Sheinberg, & Curran, 2008) and bird stimuli (Scott, Tanaka, Sheinberg, & Curran, 2006; Tanaka et al., 2005). Research also disclosed the expertise effect on N170 using artificial stimuli including Blobs (Curran, Tanaka, & Weiskopf, 2002) and Greebles (Rossion, Gauthier, Goffaux, Tarr, & Crommelinck, 2002; Rossion, Kung, & Tarr, 2004) and with non-object letter symbols, including Japanese (Maurer et al., 2008) and Chinese characters (Fan et al., 2015; Qi et al., 2016). In summary, EEG studies suggest that, similar to face perception (Hinojosa et al., 2015; Richler & Gauthier, 2014; Towler et al., 2017), visual expertise modifies the temporal aspects of information processing related with an enhanced N170 component for trained or domain-specific objects.
In 1997, Kanwisher and colleagues located a brain region in the fusiform gyrus that is strongly activated when humans view faces. This region was called the fusiform face area (FFA). Two years later, in 1999, Gauthier, Tarr, Anderson, Skudlarski, and Gore demonstrated that the FFA is not only activated when viewing faces but also indicates the level of expertise with artificial objects (in this case Greebles). The assumption was that the selectivity of FFA reflects a more generalized form of visual perceptual expertise that is not intrinsically specific or restricted to processing face stimuli (Tarr & Gauthier, 2000). This assumption was confirmed with bird (Gauthier et al., 2000), car (Gauthier et al., 2000), and artificial stimuli (Gauthier et al., 1999). However, an early criticism of these studies was that these stimuli were similar to faces: Indeed, parts of Greebles evoke resemblance to faces, birds have faces, and also cars, at least in three-quarter frontal views, resemble faces (Kanwisher, 2000; Grill-Spector, Knouf, & Kanwisher, 2004). The conclusion was, thus, that FFA activation was more likely the result of face similarity than object expertise. To minimize the effect of faces, Xu (2005) used side view photographs of birds and cars, and reported that visual perceptual expertise was still associated with FFA activation. Since then, a rich plethora of fMRI studies supported Gauthier’s initial assumption that visual expertise in object perception was associated with activation in the FFA independent of face similarity (Bilalić et al., 2011; Bukach, Gauthier, & Tarr, 2006; Palmeri & Gauthier, 2004; Righi, Tarr, & Kingon, 2013; but see Bartlett, Boggan, & Krawczyk, 2013, for a study that did not find differences between experts and novices in FFA activation in a chess task. In that study, artificially inverted and distorted chess stimuli were used, so it is a matter of debate if these stimuli were suitable to trace chess expertise). In recent years, the discussion around face selectivity tended to be replaced with a more recent discussion whether FFA was the only region relevant for processing familiar objects or whether visual perceptual expertise was associated with the interaction between different brain regions (e.g., Bilalić, Langner, Campitelli, Turella, & Grodd, 2015; Harel, Kravitz, & Baker, 2013; Wong & Wong, 2014). In short, there seems to be broad consensus in the field that the processing of objects involves more than just FFA. Specifically, Wong and Wong (2014, p. 308) explain that “perceptual expertise researchers have been considering the interaction between perceptual and cognitive processing as an important component in understanding perceptual expertise for different objects. It is therefore unnecessary to create the debate between the so-called “perceptual view” and “interactive view” of expert object recognition, as the interaction between perceptual and cognitive processing has been well accommodated in perceptual expertise research.” Overall, studies using fMRI demonstrated that when experts view domain-specific stimuli, the FFA and other brain regions are activated. The precise location of these “other” brain regions and their particular interaction patterns with FFA, however, are still under investigation.
Table 1
Studies examining neural correlates of expertise differences
with medical images as stimuli
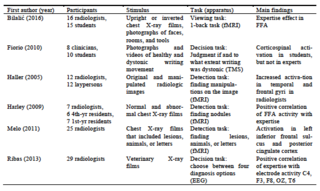
Gegenfurtner, Siewiorek, Lehtinen, and Säljö (2013) reviewed the literature on visual expertise in relation to medical image diagnosis and identified three of 21 studies that examined neural correlates of expert-novice differences when inspecting medical visualizations (Haller & Radue, 2005; Fiorio et al., 2010; Harley et al., 2009). Since the review of Gegenfurtner et al. (2013), two additional studies were published that addressed the neural basis of visual perceptual expertise in medicine (Bilalić et al., 2016; Ribas et al., 2013). We briefly review these studies here, together with an additional paper (Melo et al., 2011) that examined the neural correlates of radiologists’ diagnoses. Although Melo et al. (2011) did not analyse the effect of expertise, the study is relevant in the current context as it discusses the involvement of brain regions when deriving diagnoses from medical visualizations. Table 1 offers an overview of the six studies.
Bilalić and colleagues (2016) asked radiologists and medical students to indicate if the current stimulus they were seeing was the same as the previous one. Stimuli were chest X-ray films that were either presented in upright position or rotated by 180° (inverted), as well as stimuli including photographs of faces, rooms, and tools. The findings suggest that the FFA of radiologists compared to medical students was more sensitive in differentiating upright or rotated X-ray films from the photographs showing rooms and tools. Bilalić et al. (2016) conclude that the FFA activation was likely associated with the level of participant expertise effect. Also Harley and colleagues (2009) found a positive correlation between FFA activation and the visual expertise of radiologists. However, Harley et al. (2009) also reported that activity in the right FFA did not differ between radiologists and first-year residents looking at radiological images. Haller and Radue (2005) presented radiologic images (computer tomography scans, magnetic resonance images, and ultrasound pictures) that were either original or manipulated to radiologists and non-radiologists. The participants were asked to decide if the presented images were original or manipulated. The group of radiologists showed significantly stronger activation than the group of non-radiologists in the bilateral middle and inferior temporal gyrus, bilateral medial and middle frontal gyrus, and left superior and inferior frontal gyrus—regions that are allegedly associated with visual attention and memory retrieval (Wager & Smith, 2003). Haller and Radue’s (2005) study is interesting because it is the first to indicate that different brain regions interact when experts visually process medical images. The findings of Melo et al. (2011) and Ribas et al. (2013) further support this notion. Particularly, using EEG, Ribas and colleagues (2013) report that participations with higher levels of expertise had more electric activity compared to participants with lower levels of expertise. Fiorio et al. (2010) used transcranial magnetic stimulation (TMS) to examine how participants differed when viewing photographs and short video sequences of healthy and dystonic writing. Briefly, in TMS, a magnetic field generator is placed in close proximity to the head of a participant in order to evoke electric currents in brain areas (for introductions to TMS, see Walsh & Cowey, 2000; Ward, 2006). The authors showed that “observation of pathological actions differently modulates the viewer’s motor resonant system, depending on previous knowledge, visual expertise, and ability to recognize sub-optimal movement kinematics” (p. 698). Fiorio and colleagues (2010) used dynamic stimuli, which is still rare in the field of cognitive-neuroscience methods applied to medical diagnosis.
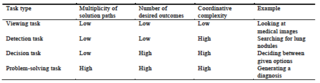
On the basis of the studies reviewed here, it seems safe to conclude that expertise in medical diagnosis cannot be located in and isolated to a single brain area but, instead, expertise seems to be associated with changes in activation in a multitude of neural regions as a function of experience, amount of training, and knowledge structures. We should note, however, that this interpretation is contingent on the level of task complexity in the original studies. It seems likely that more brain regions are activated when the task is more complex, while many studies employ simplified versions of the task of medical image diagnosis. The six studies reviewed in Table 1 differ in their task complexity. The complexity of the employed tasks was categorized following the four-level model of task complexity in the comprehension of visualizations (Gegenfurtner et al., 2011) shown in Table 2. This model defines task complexity on the basis of contextual demands that differ as a function of the number of desired outcomes, the multiplicity of paths to attain desired outcomes, and the coordinative complexity of informational cues in the task material while moving toward task completion. The reviewed studies include one viewing task, in which participants had to say if they had just seen the same image (Bilalić et al., 2016); three detection tasks, in which participants were asked to search for an abnormality or specific target within the image (Haller & Radue, 2005; Harley et al., 2009; Melo et al., 2011); and two decision tasks, in which the participants had to choose among a given set of options (Fiorio et al., 2010; Ribas et al., 2013). The study by Fioro et al. (2010) is the only one using TMS and the study by Ribas et al. (2013) the only one using EEG; thus, findings from these two studies cannot easily be compared to the other four studies using fMRI. Somewhat surprisingly, to date, no study has asked participants to produce a full diagnosis from a presented visual material, perhaps because tasks inside a magnetic resonance scanner are kept deliberately simple and diagnostic problem-solving tasks would be too complex; even a simple blink causes severe artifacts in electroencephalograms. Typing is practically impossible, and speaking might be hard to record due to the noise made by the scanner. Furthermore, if the task is too complex in comparison to the control task, this might lead to differences in brain activation that are so widespread that it might no longer be able to meaningfully interpret them. This explains perhaps the scarcity of cognitive-neuroscience studies in medical image diagnosis relative to its wide application in the visual perceptual expertise literature.
Table 2
Four-level model of task complexity in the comprehension of
visualizations (adapted from Gegenfurtner, Lehtinen, &
Säljö, 2011)
If we compare the studies using medical images as experimental stimuli (reviewed in Table 1) with the wider visual perceptual expertise literature and their findings of FFA activation and the enhanced N170 component, it is evident that the expertise effect in FFA was partially confirmed with medical images (Bilalić et al., 2016; Harley et al., 2009). An increased N170 component has not yet been systematically addressed. It is very encouraging that, since our review some years ago (Gegenfurtner et al., 2013), more and more cognitive-neuroscience studies using fMRI or EEG emerge that address medical image diagnosis. We do expect that future research will proliferate in this area in an attempt to replicate FFA activation and N170 enhancement as neural correlates of visual expertise in the medical domain. These studies will help us understand how medical expertise changes temporal and spatial brain activation patterns associated with the diagnosis of medical visualizations.
After reviewing typical research questions and experimental stimuli, describing EEG and fMRI, and reporting the current state of the neural correlates of visual perceptual expertise, this section will now elaborate on the advantages and limitations of cognitive-neuroscience research in the current context. What are the benefits of using fMRI and EEG? What are caveats of these methodologies? And what are directions for future research that originate from this review?
The benefits of applying cognitive-neuroscience methods in research on visual perceptual expertise relate to the extension of behavioural research, high temporal and spatial sensitivity, and high levels of control. We elaborate on each of these benefits in turn. First, cognitive neuroscience can extend behavioural research. In particular, cognitive neuroscience affords different units and levels of analysis; these, in turn, make visible some of the neural correlates underlying cognitive processes that would not be accessible with behavioural measures (Ansari, De Smedt, & Grabner, 2012). Framing this triangulation, Stern and Schneider (2010) introduced the metaphor of a digital road map: with cognitive neuroscience, researchers can zoom in to the neural levels of cognition and perception and examine processes inside the human brain. If researchers are interested in these processes, EEG and fMRI offer measures that can unveil neural activation as the basis for observable expertise differences (Gegenfurtner et al., 2013; Gruber et al., 2010).
Another advantage of many cognitive-neuroscience methods is their very high temporal and spatial resolution. More precisely, EEG takes measures in the range of milliseconds. Thus, if we are interested in the temporal aspects of expert performance, then time-sensitive EEG is a very suited method, especially if measured in parallel with pupillometry (Szulewski, Gegenfurtner, Howes, Sivilotti, & Van Merriënboer, 2017) and eye tracking (Holmqvist, Nyström, Anderson, Dewhurst, Jarodzka, & Van de Weijer, 2011; Jarodzka, Jaarsma, & Boshuizen, 2015). In contrast, fMRI has a unique capability in locating very precisely the brain regions that are active, e.g., when participants of varying levels of expertise interpret complex images. If research aims to uncover where and when neural activity occurs during expert performance, then EEG and fMRI (at best in combination) are two very powerful, non-invasive methodological tools.
Finally, because of the extremely high sensitivity of EEG (temporal) and fMRI (spatial), experiments in cognitive neuroscience are typically very controlled. These levels of control afford high levels of external validity (Ansari et al., 2012; De Smedt, 2014). Because researchers invest a considerable amount of time and energy in securing experimental control, including a strict selection of participants (for example: only right-handed people) and carefully filtered stimulus materials (exemplarily reflected in the huge effort of creating Greebles), findings from EEG and fMRI often result in stable, generalizable inferences.
These generalizable inferences can inform researchers when developing theories of visual expertise. Because cognitive-neuroscience methods have high levels of temporal and spatial sensitivity, as well as experimental control, neural correlates of visual expertise can be used in theory testing and development (Bilalić et al., 2015). Neuroscientific findings thus have the potential to inform expertise research in two ways. First, they can be used to test the predictive validity of existing models and theories, for example on how expertise develops in novices (Kok, De Bruin, Robben, & Van Merriënboer, 2012; Van Geel, Kok, Dijkstra, Robben, & Van Merriënboer, 2017), intermediates (Boshuizen & Schmidt, 1992; Ericsson & Lehmann, 1992), and experts (Gegenfurtner, 2013; Gegenfurtner, Nivala, Lehtinen, & Säljö, 2009). Second, they can be used to develop novel theories to account for expertise differences revealed by methods of cognitive-neurosciences; differences that would have remained unobservable with behavioral methods alone (Bilalić et al., 2015).
No method comes without limitations. Powerful and elegant as cognitive neuroscience may appear, its methodology also includes different costs that can compromise the available evidence. Caveats include the temporal and spatial resolution, ecological validity, a reductive bias, and limited implications for educational practice. First, and perhaps surprisingly, the extreme sensitivity of EEG and fMRI measures, positive on one side, introduces of course a number of limitations to the experimental setup. For example, in EEG research, already the slightest motions like a blink or moving the nostrils creates severe data artifacts. Research projects thus often lose a considerable amount of data because participants had not been motionless enough while their neural activity was recorded (Ansari et al., 2012; De Smedt, 2014). This is particularly detrimental if we consider the financial costs of data collection and if we consider that cognitive neuroscience often works with small sample sizes. To cover for this data loss, even higher and stricter experimental controls are developed, implemented, and employed. The fact that EEG and fMRI are so sensitive to small motions causing artefacts means that the ecological validity is easily compromised: the sensitivity to motion restricts the possibilities for ecologically valid experiments.
This is related to compromises in the ecological validity of cognitive neuroscience experiments. Experts are not typically motionless or work inside the tube of a 3-tesla MRI scanner. It is thus a matter of debate to which extent cognitive neuroscience can reflect the complexity of processes and practices associated with real-world expertise. Do we force experts to act in too artificial ways? Can we capture how experts diagnose a patient case when we show them a chest X-ray in a rotated, blurred, or otherwise distorted mode, for the duration of only a few seconds? The high level of experimental control, that is clearly a benefit of cognitive neuroscience, comes at the same time with limitations to ecological validity. The limited ecological validity is also associated with tasks that are typically used. Instead of complex problem-solving tasks that would reflect medical diagnosis, many of the reviewed studies employed lower levels of task complexity (Gegenfurtner et al., 2011). Furthermore, study participants are asked to complete these tasks repeatedly in longer sessions to get readable signals, which can further compromise ecological validity. This is in line with De Smedt’s (2014) observation that tasks used in neuroscience “need to be very elementary, because the larger the number of cognitive processes in a particular task, the more difficult it will be to disentangle these cognitive processes physiologically.”
Cognitive neuroscience is interested in the neural correlates of behavior, cognition, emotion etc. (Squire et al., 2013; Stern & Schneider, 2010; Ward, 2006). Epistemologically, from a neuroscience perspective, visual perceptual expertise tends to be reduced to changes in electrical activity or blood flow. While this can render fascinating findings, other important ingredients of expert performance are ignored. Certainly, all research is reductionist (Lehtinen, 2012; Säljö, 2009). One must make decisions what to measure because we simply cannot account for all relevant aspects in a single study, as interesting these aspects may be (Damşa et al., 2017). Focusing on neural levels does not imply that we uncover the basis of human learning. One could easily argue that the basis is the social context within which we are situated (Gegenfurtner & Szulewski, 2017; Säljö, 2009). In describing this reductive bias, Lehtinen (2012) notes: “Because of the impressive technical development of brain research during the last two decades (…) many neuroscientists have quite a strong tendency towards downwards reductionism (emphasis in the original). This reductionism stems from the idea that research registering brain processes with complex technical tools finally opens up a real scientific approach to learning research.” Cognitive neuroscientists are well aware that EEG and fMRI are just two among the many other methods of learning research (e.g., De Smedt, 2014).
This reductive bias is not only associated with limitations in how expertise is measured and methodically approximated; it also signals limitations in how expertise and performance are theorized and conceptually framed (Lehtinen, 2012; Säljö, 2009; Siewiorek & Gegenfurtner, 2010). More specifically, theories of visual expertise that are exclusively grounded on neuroscientific evidence risk to de-emphasize other facets of how expert performance is enacted and displayed in real-world activities and practices (Gibson, 1986; Goodwin, 1994; see also De Bruin, 2017; Gegenfurtner et al., 2017). This risk is of course inherent in all mono-method designs, largely because single method studies capture a limited number of units of analysis. Conversely, the combination of approaches in mixed-method or multi-method designs allow for the triangulation of units of analyses, which can inform a theory of visual perceptual expertise that encompasses different analytic levels beyond what is evident from single method approaches like EEG, TMS, or fMRI. While the benefits of bridging methods of expertise research are clear, methods are always part of a scientific community. These communities have agency as political actors and “defend” their methods against the influences of concurrent academic realms (Al Lily, Foland, Stoloff, Gogus, Erguvan, Awshar, et al., 2017), so it will be an interesting observation to see if and to what extent expertise researchers will (continue to) embrace methodological triangulations and combine cognitive neurosciences with other method approaches in their studies for the purpose of theory development.
Related to that is a false belief that findings from EEG and fMRI would be directly applicable and informative for re-designing learning environments and curricula. Educational neuroscientists work hard to deemphasize the hopes that many practitioners have when they read that finally, once we understand the brain, we understand how learning, expertise, and education “work”. Ansari and colleagues (2012) write that “the most obvious question a teacher may ask is, ‘How will I be able to apply this knowledge?’ There is, in our view, no reason to expect that neuroimaging research, will determine directly how teaching should take place. This is considered by many ‘a bridge too far’”. Thus, cognitive or educational neuroscience may have a very limited impact on educational practices. Does this mean we should not conduct this kind of research? Certainly not; but we should, perhaps, rethink our expectations about what neuroscience measures can do (Ansari et al., 2012; De Smedt, 2014; Lehtinen, 2012; Säljö, 2009; Stern & Schneider, 2010).
Examining the neural correlates of visual expertise is a fascinating endeavour. This review has identified a small, still limited number of studies that examined how visual perceptual expertise in medical image diagnosis correlates with EEG, fMRI, and TMS measures. What are directions for future research that follow from this review? First, all but one of the reviewed studies in Table 1 used static pictures. Only Fiorio and colleagues (2010) used video sequences as stimuli. We thus recommend exploring and testing if and to what extent neuroscience-based visual perceptual expertise research can use dynamic stimuli. Second, future research can make more use of EEG as well as other neuroscience approaches such as TMS or MEG to study the neural correlates of visual expertise in medical image diagnosis. Another possible direction for future research is the combination of neuroscience methods with other online measures of expertise, including eye tracking and pupillometry (Holmqvist et al., 2011; Gegenfurtner & Seppänen, 2013; Kok et al., 2012; Szulewski et al., 2017) if the constraints of different temporal scales can be accommodated for. Such combinations would be interesting theoretically as a means to inquire how eye movements and neural activity correlate in expert diagnostic reasoning. Fourth, implications of cognitive-neurosciences for education and training need to be explored. To what extent can clinical practitioners and medical educators benefit from neuroscientific measures? This is a question that applies to the field of medical image perception more generally and is not exclusive to cognitive-neurosciences; for example, also eye tracking used to be criticized for not being relevant enough to medical education and training, but has demonstrated its benefits in the form of eye movement modeling examples (Jarodzka, Balslev, Holmqvist, Nyström, Scheiter, Gerjets, et al., 2012; Seppänen & Gegenfurtner, 2012). It remains to be seen in future research if, and how, a similar approach can be developed for functional imaging. We should note, however, that cognitive-neurosciences are useful methods in addition to instructional design studies: while design studies reveal what works, neural correlates can indicate why it works (Gegenfurtner et al., 2013; Kok, Van Geel, Van Merriënboer, & Robben, 2017). Finally, EEG and fMRI are measures into the temporal and spatial configurations of visual perceptual expertise. These measures should be incorporated into existing theory frameworks of visual perceptual expertise to advance our conceptual understanding of how experts, intermediates, or novices comprehend medical visualizations.
As noted at the outset, if we assume that individual differences in visual expertise are reflected in differences in the brain, then cognitive neuroscience methods can be used to examine the neural correlates of the experts’ visual skills. These methods can complement other methodologies interested in how experts in medical disciplines form their diagnoses. This review summarized research on visual perceptual expertise and described which research questions were typically asked, which stimuli and functional neuroimaging methods were frequently used, and how experts and novices differ in their neural representations (e.g., with respect to activation within the FFA). We also outlined some of the benefits, limitations, and future directions of cognitive-neuroscience research as they apply to the comprehension of (medical) visualizations. This methodological review closes with the hope that interested researchers, who are perhaps yet inexperienced with cognitive neuroscience, will find this paper a useful introduction into the neural correlates of visual expertise.
Al Lily, A., Foland, D., Stoloff, D., Gogus, A., Erguvan, I. D.,
Awshar, M. T., et al. (2017). Academic domains as political
battlegrounds: A global enquiry by 99 academics in the fields of
education and technology. Information Development.
doi:10.1177/0266666916646415
Ansari, D., De Smedt, B., & Grabner, R. (2012). Neuroeducation
- a critical overview of an emerging field. Neuroethics, 5,
105-117. doi:10.1007/s12152-011-9119-3
Bartlett, J., Boggan, A. L., & Krawczyk, D. C. (2013).
Expertise and processing distorted structure in chess. Frontiers
in Human Neuroscience, 7, 825. doi:10.3389/fnhum.2013.00825
Bentin, S., Allison, T., Puce, A., Perez, E., & McCarthy, G.
(1996). Electrophysiological studies of face perception in humans.
Journal of Cognitive Neuroscience, 8, 551-565.
doi:10.1162/jocn.1996.8.6.551
Bilalić, M., Grottenthaler, T., Nägele, T., & Lindig, T.
(2016). The faces in radiologic images: Fusiform face area
supports radiological expertise. Cerebral Cortex, 26, 1004-1014.
doi:10.1093/cercor/bhu272
Bilalić, M., Langner, R., Campitelli, G., Turella, L., &
Grodd, W. (2015). Editorial: Neural implementation of expertise.
Frontiers in Human Neuroscience. doi:10.3389/fnhum.2015.00545
Bilalić, M., Langner, R., Ulrich, R., & Grodd, W. (2011).
Many faces of expertise: Fusiform face area in chess experts and
novices. Journal of Neuroscience, 31, 10206-10214.
doi:10.1523/JNEUROSCI.5727 -10.2011
Boshuizen, H. P. A., & Schmidt, H. G. (1992). On the role of
biomedical knowledge in clinical reasoning by experts,
intermediates, and novices. Cognitive Science, 16, 153-184.
doi:10.1207/s15516709cog1602_1
Bukach, C. M., Gauthier, I., & Tarr, M. J. (2006). Beyond
faces and modularity: the power of an expertise framework. Trends
in Cognitive Science, 10, 159-166. doi:10.1016/j.tics.2006.02.004
Curran, T., Tanaka, J. W., & Weiskopf, D. M. (2002). An
electrophysiological comparison of visual categorization and
recognition memory. Cognitive, Affective, and Behavioral
Neuroscience, 2, 1-18. doi:10.3758/CABN.2.1.1
Damşa, C. I., Froehlich, D. E., & Gegenfurtner, A. (2017).
Reflections on empirical and methodological accounts of agency at
work. In M. Goller & S. Paloniemi (Eds.), Agency at work: An
agentic perspective on professional learning and development. New
York: Springer.
De Bruin, A. B. H. (2017). The potential of neuroscience for
health sciences education: towards convergence of evidence and
resisting seductive allure. Advances in Health Sciences Education.
doi:10.1007/s10459-016-9733-2
De Smedt, B. (2014). Advances in the use of neuroscience methods
in research on learning and instruction. Frontline Learning
Research, 6, 7-14. doi:10.14786/flr.v2i4.115
Fan, C., Chen, S., Zhang, L., Qi, Z., Jin, Y., Wang, Q., et al.
(2015). N170 changes reflect competition between faces and
identifiable characters during early visual processing.
NeuroImage, 110, 32-38. doi:10.1016/j.neuroimage.2015.01.047
Fiorio, M., Cesari, P., Bresciani, M. C., & Tinazzi, M.
(2010). Expertise with pathological actions modulates a viewer’s
motor system. Neuroscience, 167, 691-699.
doi:10.1016/j.neuroscience.2010.02.010.
Gauthier, I., & Curby, K. M. (2005). A perceptual traffic jam
on highway N170. Current Directions in Psychological Science, 14,
30-33. doi:10.1111/j.0963-7214.2005.00329.x
Gauthier, I., Skudlarski, P., Gore, J. C., Anderson, A. W. (2000).
Expertise for cars and birds recruits brain areas involved in face
recognition. Nature Neuroscience, 3, 191-197. doi:10.1038/72140
Gauthier, I., Tarr, M. J., Anderson, A. W., Skudlarski, P., &
Gore, J. C. (1999). Activation of the middle fusiform ’face area’
increases with expertise in recognizing novel objects. Nature
Neurosciences, 2, 568-573. doi:10.1038/9224
Gauthier, I., Williams, P., Tarr, M. J., & Tanaka, J. (1998).
Training ’greeble’ experts: a framework for studying expert object
recognition processes. Vision Research, 38, 2401-2428.
doi:10.1016/S0042-6989(97)00442-2
Gegenfurtner, A. (2013). Transitions of expertise. In J. Seifried
& E. Wuttke (Eds.), Transitions in vocational education (pp.
305-319). Opladen: Budrich.
Gegenfurtner, A., Kok, E., Van Geel, K., De Bruin, A., Jarodzka,
H., Szulewski, A., & Van Merriënboer, J. J. G. (2017). The
challenges of studying visual expertise in medical image
diagnosis. Medical Education, 51, 97-104. doi:10.1111/medu.13205
Gegenfurtner, A., Lehtinen, E., & Säljö, R. (2011). Expertise
differences in the comprehension of visualizations: A
meta-analysis of eye-tracking research in professional domains.
Educational Psychology Review, 23, 523-552.
doi:10.1007/s10648-011-9174-7
Gegenfurtner, A., Nivala, M., Säljö, R., & Lehtinen, E.
(2009). Capturing individual and institutional change: Exploring
horizontal versus vertical transitions in technology-rich
environments. In U. Cress, V. Dimitrova, & M. Specht (Eds.),
Learning in the synergy of multiple disciplines. Lecture Notes in
Computer Science (pp. 676-681). Berlin: Springer.
doi:10.1007/978-3-642-04636-0_67
Gegenfurtner, A., & Seppänen M. (2013). Transfer of expertise:
An eye-tracking and think-aloud study using dynamic medical
visualizations. Computers & Education, 63, 393-403.
doi:10.1016/j.compedu.2012.12.021
Gegenfurtner, A., Siewiorek, A., Lehtinen, E., & Säljö, R.
(2013). Assessing the quality of expertise differences in the
comprehension of medical visualizations. Vocations and Learning,
6, 37-54. doi: 10.1007/s12186-012-9088-7
Gegenfurtner, A., & Szulewski, A. (2016). Visual expertise and
the Quiet Eye in sports – comment on Vickers. Current Issues in
Sport Science, 1, 108. doi:10.15203/CISS_2016.108
Gegenfurtner, A., & Van Merriënboer, J. J. G. (2017).
Methodologies for studying visual expertise. Frontline Learning
Research.
Gibson, J. (1986). The ecological approach to visual perception.
New York: Psychology Press.
Goodwin, C. (1994). Professional vision. American Anthropologist,
96, 606-633. doi:10.1525/aa.1994.96.3. 02a00100
Grill-Spector, K., Knouf, N., & Kanwisher, N. (2004) The
fusiform face area subserves face perception, not generic
within-category identification. Nature Neuroscience, 7, 555-562.
doi:10.1038/nn1224
Gruber, H., Jansen, P., Marienhagen, J., & Altenmüller, E.
(2010). Adaptations during the acquisition of expertise. Talent
Development & Excellence, 2, 3-15.
Haller, S., & Radue, E. W. (2005). What is different about a
radiologist’s brain? Radiology, 236, 983-989.
doi:10.1148/radiol.2363041370
Harel, A., Kravitz D., & Baker C. I. (2013). Beyond perceptual
expertise: revisiting the neural substrates of expert object
recognition. Frontiers in Human Neuroscience, 7, 885.
doi:10.3389/fnhum.2013.00885
Harley, E. M., Pope, W. B., Villablanca, J. P., Mumford, J., Suh,
R., Mazziotta, J. C., et al. (2009). Engagement of fusiform cortex
and disengagement of lateral occipital cortex in the acquisition
of radiological expertise. Cerebral Cortex, 19, 2746-2754.
doi:10.1093/cercor/bhp051
Helle, L., Nivala, M., Kronqvist, P., Gegenfurtner, A., Björk, P.,
& Säljö, R. (2011). Traditional microscopy instruction versus
process-oriented virtual microscopy instruction: A naturalistic
experiment with control group. Diagnostic Pathology, 6, S81-S89.
doi:10.1186/1746-1596-6-S1-S8
Hinojosa, J. A., Mercado, F., & Carretié (2015). N170
sensitivity to facial expression: A meta-analysis. Neuroscience
& Biobehavioral Reviews, 55, 498-509.
doi:10.1016/j.neubiorev.2015.06.002
Holmqvist, K., Nyström, N., Andersson, R., Dewhurst, R., Jarodzka,
H., & Van de Weijer, J. (2011). Eye tracking: A comprehensive
guide to methods and measures. Oxford: Oxford University Press.
Jarodzka, H., Balslev, T., Holmqvist, K., Nyström, M., Scheiter,
K., Gerjets, P., et al. (2012). Conveying clinical reasoning based
on visual observation via eye-movement modelling examples.
Instructional Science, 40, 813-827. doi:10.1007/s11251-012-9218-5
Jarodzka, H., Jaarsma, T., & Boshuizen, H. P. A. (2015). In my
mind: How situation awareness can facilitate expert performance
and foster learning. Medical Education, 49, 854-856.
doi:10.1111/medu.12791
Kanwisher, N. (2000). Domain specificity in face perception.
Nature Neuroscience, 3, 759-763. doi: 10.1038/77664
Kanwisher, N., McDermott, J., & Chun, M. M. (1997). The
fusiform face area: A module in human extrastriate cortex
specialized for face perception. Journal of Neuroscience, 17,
4302-4311.
Kok, E. M., De Bruin, A. B. H., Robben, S. G. F., & Van
Merriënboer, J. J. G. (2012). Looking in the same manner but
seeing it differently: Bottom-up and expertise effects in
radiology. Applied Cognitive Psychology, 26, 854-862.
doi:10.1002/acp.2886
Kok, E. M., Van Geel, K., Van Merriënboer, J. J. G., & Robben,
S. G. F. (2017). What we do and do not know about teaching medical
image interpretation. Frontiers in Psychology, 8, 309.
doi:10.3389/fpsyg.2017.00309
Lehtinen, E. (2012). Learning of complex competences: On the need
to coordinate multiple theoretical perspectives. In A. Koskensalo,
J. Smeds, R. de Cillia, & Á. Huguet (Eds.), Language:
Competencies - change - contact (pp. 13-27). Berlin: LIT.
Maurer, U., Zevin, J. D., & McCandliss, B. D. (2008)
Left-lateralized N170 effects of visual expertise in reading:
evidence from Japanese syllabic and logographic scripts. Journal
of Cognitive Neuroscience 20, 1878-1891. doi:10.1162/
jocn.2008.20125
Melo, M., Scarpin, D. J., Amaro, E., Passos, R. B., Sato, J. R.,
Friston, K. J., et al. (2011). How doctors generate diagnostic
hypotheses: A study of radiological diagnosis with functional
magnetic resonance imaging. PLoS ONE, 6, e28752.
doi:10.1371/journal.pone.0028752.
Nishimura, M., & Maurer, D. (2008). The effect of
categorisation on sensitivity to second-order relations in novel
objects. Perception, 37, 584-601. doi:10.1068/p5740
Op de Beeck, H. P., Baker, C. I., DiCarlo, J. J., & Kanwisher,
N. G. (2006). Discrimination training alters object
representations in human extrastriate cortex. Journal of
Neuroscience, 26, 13025-13036. doi:10.1523/JNEUROSCI.2481-06.2006
Qi, Z., Wang, X., Hao, S., Zhu, C., He, W., & Luo, W. (2016).
Correlations of electrophysiological measurements with
identification levels of ancient Chinese characters. PLoS ONE, 11,
e0151133. doi: 10.1371/journal.pone.0151133
Palmeri, T. J., & Gauthier, I. (2004). Visual object
understanding. Nature Reviews Neuroscience, 5, 291-303.
doi:10.1038/nrn1364
Ribas, L. M., Rocha, F. T., Siqueira Ortega, N. R., Freitas de
Rocha, A., & Massad, E. (2013). Brain activity and medical
diagnosis: an EEG study. BMC Neuroscience, 14, 109.
doi:10.1186/1471-2202-14-109
Richler, J. J., & Gauthier, I. (2014). A meta-analysis and
review of holistic face processing. Psychological Bulletin, 140,
1281-1302. doi:10.1037/a0037004
Righi, G. R., Tarr, M., & Kingon, A. (2013).
Category-selective recruitment of the fusiform gyrus with chess.
In J. Staszewski (Ed.), Expertise and skill acquisition: the
impact of William G. Chase (pp. 261-280). New York: Taylor &
Francis.
Rossion, B., Gauthier, I., Goffaux, V., Tarr, M. J., &
Crommelinck, M. (2002). Expertise training with novel objects
leads to left-lateralized facelike electrophysiological responses.
Psychological Science, 13, 250-257. doi:10.1111/1467-9280.00446
Rossion, B., Kung, C.-C., & Tarr, M. J. (2004). Visual
expertise with nonface objects leads to competition with the early
perceptual processing of faces in the human occipitotemporal
cortex. Proceedings of the National Academy of Sciences, 101,
14521-14526. doi:10.1073/pnas.0405613101
Säljö, R. (2009). Learning, theories of learning, and units of
analysis in research. Educational Psychologist, 44, 202-208.
doi:10.1080/00461520903029030
Scott, L. S., Tanaka, J. W., Sheinberg, D. L., & Curran, T.
(2006). A reevaluation of electrophysiological correlates of
expert object processing. Journal of Cognitive Neuroscience, 18,
1453-1465. doi:10.1162/jocn.2006.18.9.1453
Scott, L. S., Tanaka, J. W., Sheinberg, D. L., & Curran, T.
(2008). The role of category learning in the acquisition and
retention of perceptual expertise: A behavioral and
neurophysiological study. Brain Research, 1210, 204-215.
doi:10.1016/j.brainres.2008.02.054
Seppänen, M., & Gegenfurtner, A. (2012). Seeing through a
teacher’s eyes improves students’ imaging interpretation. Medical
Education, 46, 1113-1114. doi:10.1111/medu.12041
Sergent, J., Ohta, S., & MacDonald, B. (1992). Functional
neuroanatomy of face and object processing. A positron emission
tomography study. Brain, 115, 15-36. doi:10.1093/brain/115.1.15
Shen, J., Mack, M. L., & Palmeri, T. J. (2014). Studying
real-world perceptual expertise. Frontiers in Psychology, 5, 857.
doi:10.3389/fpsyg.2014.00857
Siewiorek, A., & Gegenfurtner, A. (2010). Leading to win: The
influence of leadership style on team performance during a
computer game training. In K. Gomez, L. Lyons, & J. Radinsky
(Eds.), Learning in the disciplines (Vol. 1, pp. 524-531).
Chicago, IL: International Society of the Learning Sciences.
Squire, L. R., Berg, D., Bloom, F. E., Du Lac, S., Ghosh, A.,
& Spitzer, N. C. (2013). Fundamental neuroscience (4th ed.).
Oxford: Academic Press.
Stern, E., & Schneider, M. (2010). A digital road map analogy
of the relationship between neuroscience and educational research.
ZDM - The International Journal on Mathematics Education, 42,
511-514. doi:10.1007/s11858-010-0278-1
Szulewski, A., Gegenfurtner, A., Howes, D., Sivilotti, M., &
Van Merriënboer, J. J. G. (2017). Measuring physician cognitive
load: Validity evidence for a physiologic and a psychometric tool.
Advances in Health Sciences Education.
doi:10.1007/s10459-016-9725-2
Tanaka, J. W., & Curran, T. (2001). A neural basis for expert
object recognition. Psychological Science, 12, 43-47.
doi:10.1111/1467-9280.00308
Tarr, M. J., & Gauthier, I. (2000). FFA: a flexible fusiform
area for subordinate-level visual processing automatized by
expertise. Nature Neuroscience, 3, 764-769. doi:10.1038/77666
Towler, J., Fisher, K., & Eimer, M. (2017). The cognitive and
neural basis of developmental prosopagnosia. The Quarterly Journal
of Experimental Psychology, 72, 316-344.
doi:10.1080/17470218.2016.1165263
Van Geel, K., Kok, E. M., Dijkstra, J., Robben, S. G. F., &
Van Merriënboer, J. J. G. (2017). Teaching systematic viewing to
final-year medical students improves systematicity but not
coverage or detection of radiologic abnormalities. Journal of the
American College of Radiology, 14, 235-241.
doi:10.1016/j.jacr.2016.10.001
Walsh, V., & Cowey, A. (2000). Transcranial magnetic
stimulation and cognitive neuroscience. Nature Reviews
Neuroscience, 1, 73-80.
Ward, J. (2006). The student's guide to cognitive neuroscience.
New York: Psychology Press.
Wong, A. C.-N., Palmeri, T. J., & Gauthier, I. (2009).
Conditions for facelike expertise with objects. Becoming a
ziggerin expert—but which type? Psychological Science, 20,
1108-117. doi:10.1111/j.1467-9280.2009.02430.x
Wong, A. C.-N., & Wong, Y. K. (2014). Interaction between
perceptual and cognitive processing well acknowledged in
perceptual expertise research. Frontiers in Human Neuroscience, 8,
308. doi:10. 3389/fnhum.2014.00308
Xu, Y. (2005). Revisiting the role of the fusiform face area in
visual expertise. Cerebral Cortex, 15, 1234-1242.
doi:10.1093/cercor/bhi006